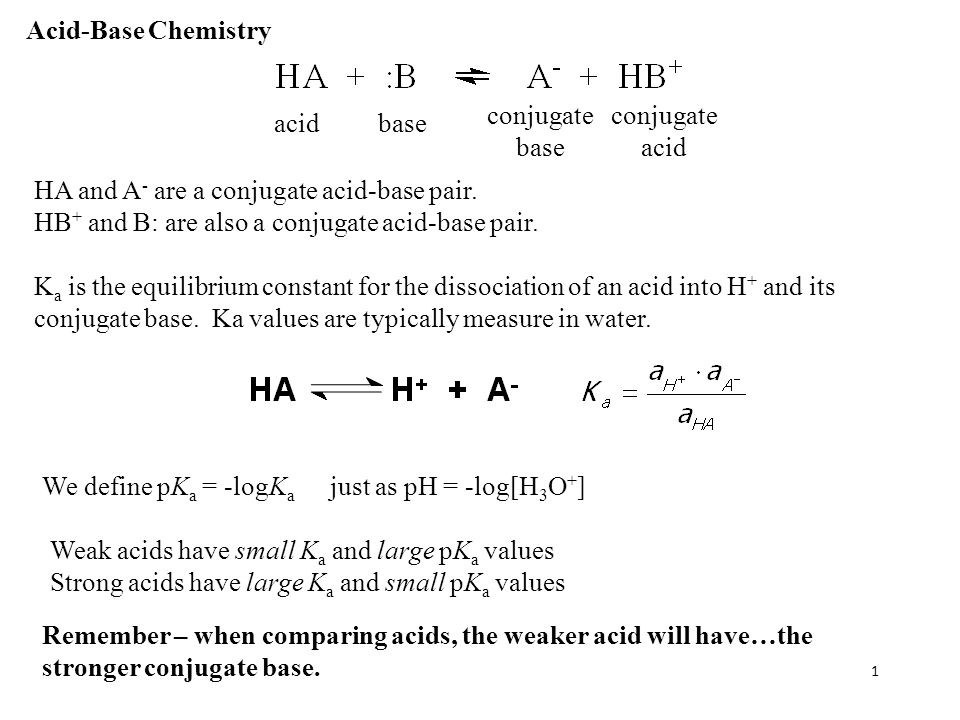
Acid-Base Chemistry K a is the equilibrium constant for the dissociation of an acid into H + and its conjugate base. Ka values are typically measure in. - ppt download

How To Calculate the PH of a Buffer Solution | Equation & Example - Video & Lesson Transcript | Study.com


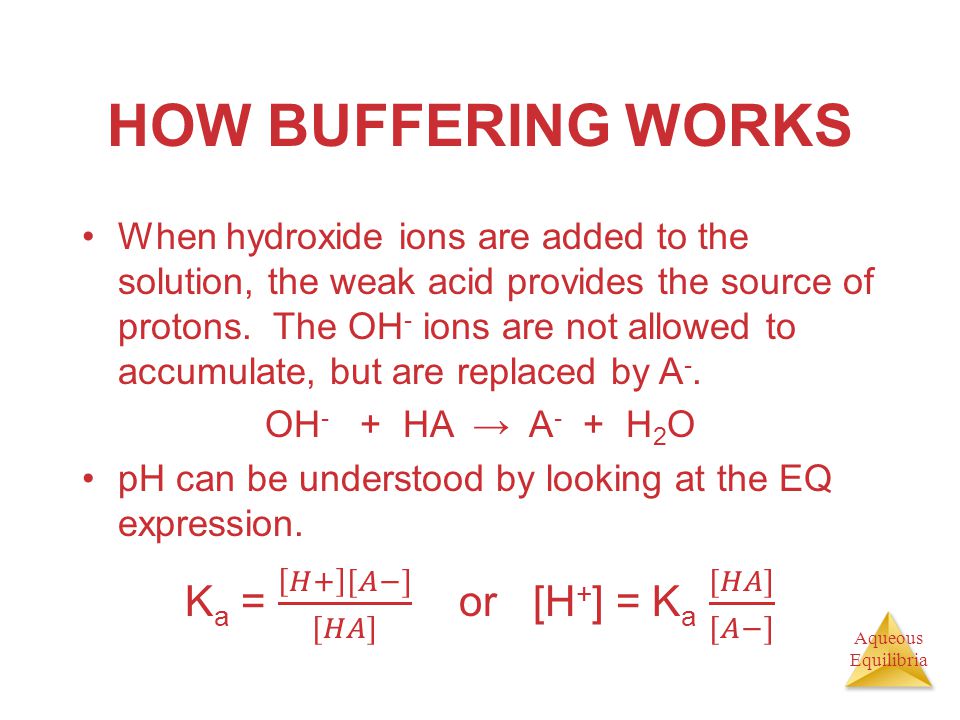



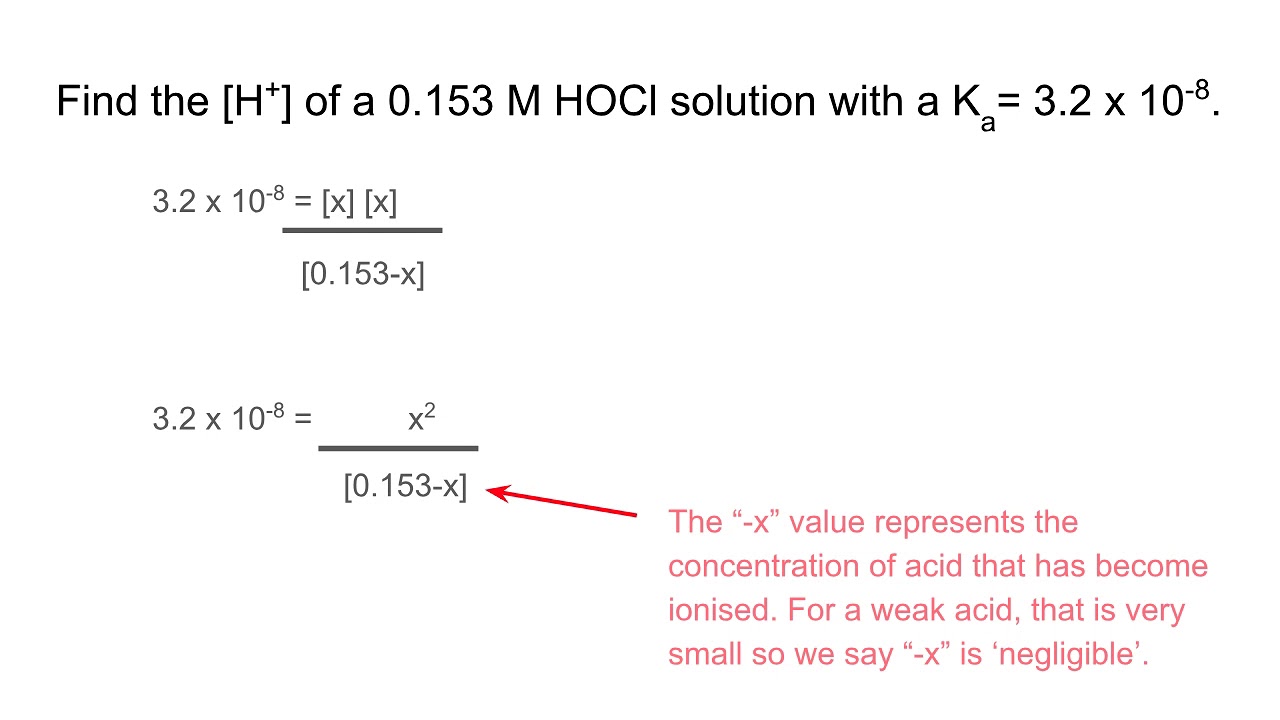






![Calculating [H+] and pH from Ka Calculating [H+] and pH from Ka](https://www.mi.mun.ca/users/pfisher/chemistry1011_134/img013.gif)